Lakewood, Colorado (Terumo BCT Sterilization Service, Inc.)
Terumo BCT Sterilization Service, Inc. is located at 11308 W. Collins Avenue, Lakewood, CO. The facility uses ethylene oxide (EtO) to sterilize medical equipment and materials.
EPA assessed the impact of EtO emissions from the Terumo BCT Sterilization Service, Inc. facility. As part of this risk assessment, we used the most recent available information about how much EtO the company emits into the air and we modeled estimated cancer risks to people living nearby. The risk assessment identified elevated cancer risk in the Lakewood community. EPA is committed to working with state and local agencies, facilities, and communities to reduce this risk.
Terumo BCT Sterilization Service, Inc.
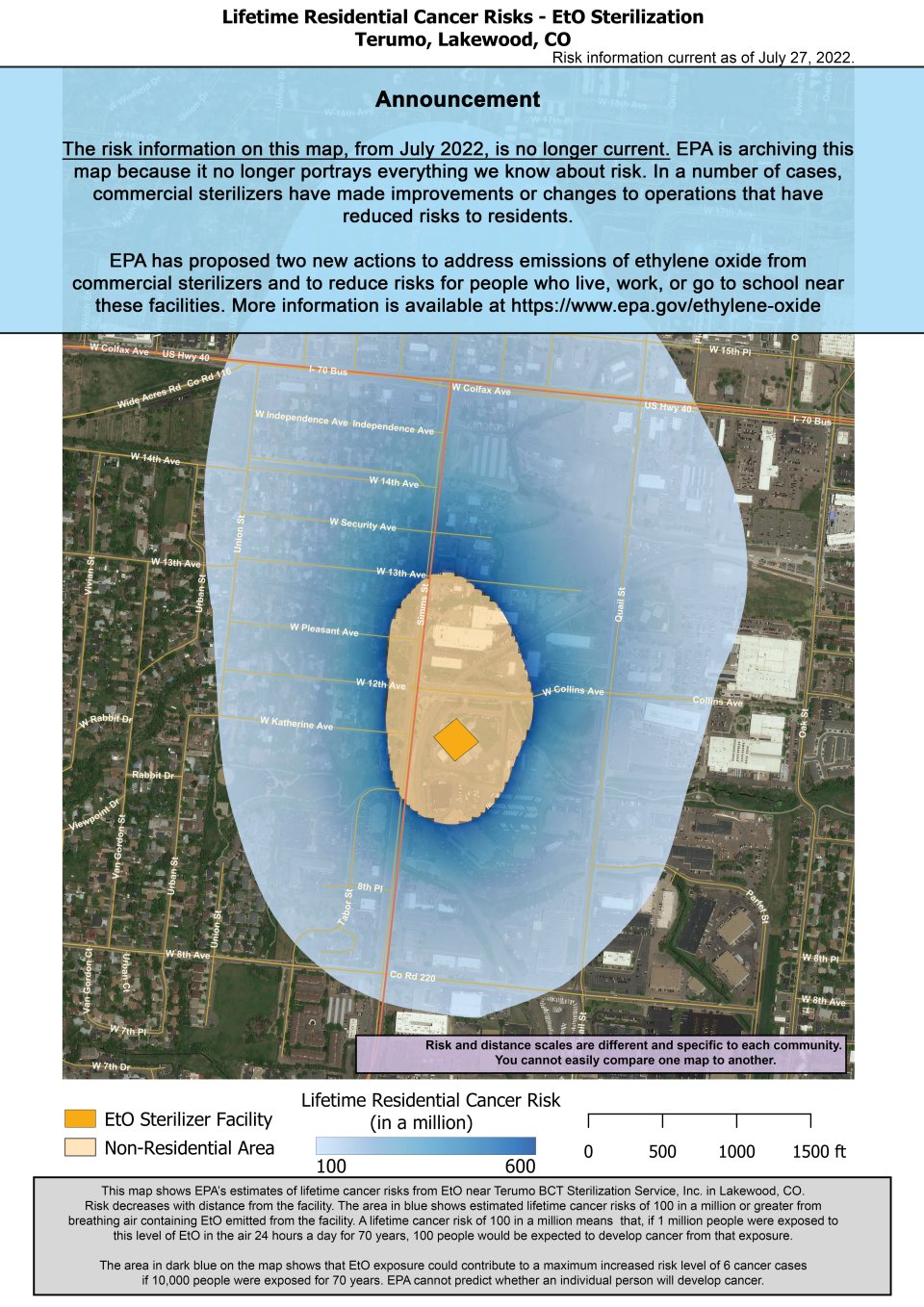
This map shows EPA’s estimates of lifetime cancer risks from EtO near Terumo BCT Sterilization Service, Inc. in Lakewood, CO. As you can see from the map risk decreases with distance from the facility.
The area in blue shows estimated lifetime cancer risks of 100 in a million or greater from breathing air containing EtO emitted from the facility (or the same as 1 additional cancer case in 10,000 people). A lifetime cancer risk of 100 in a million means that, if 1 million people were exposed to this level of EtO in the air 24 hours a day for 70 years, 100 people would be expected to develop cancer from that exposure.
The area in dark blue on the map shows that EtO exposure could contribute to a maximum increased risk level of 600 cancer cases if one million people were exposed for 70 years (or 6 in 10,000). EPA cannot predict whether an individual person will develop cancer.
View a larger version of the map and legend in a new browser tab.
For this risk assessment, we looked at excess cancer risk attributable to a single chemical, EtO. This estimated risk is in addition to the risk of developing cancer from other causes. This is a worst-case scenario that assumes a person stays in the highest risk area 24 hours a day continuously for 70 years. EPA takes this approach because we want to be protective of the most exposed and most vulnerable individuals from risk associated with EtO emissions from this facility.
Community Details
Terumo BCT is a global medical device manufacturing company with a headquarters and campus in Lakewood, Colorado. The company acquired the Lakewood location from COBE Laboratories in 2012, and the current medical sterilization facility became operational in 2000. The company currently employs 18 people at its sterilization facility and 2,000 in Colorado. The facility is not violating any current air pollution control regulatory requirements.
What EPA is Doing to Address Ethylene Oxide
Now: EPA is working with the State of Colorado to reduce emissions at Terumo BCT. EPA has provided technical support to our air agency partners as part of this work. The Agency is reviewing controls on regulated equipment and processes that emit EtO to determine whether additional air pollution controls are needed. This review includes examining new developments in practices, processes and control technologies, considering cost and feasibility, as well as addressing any previously unregulated emission points.
EPA hosted a community meeting for Lakewood-area residents on September 22, 2022 to present the results of the agency’s 2022 ethylene oxide risk modeling analysis for the Terumo BCT Sterilization Services facility. This modeling adds new information and resolution to previous activities taken by the company and state health officials in 2018 to evaluate and reduce ethylene oxide risk in the area surrounding the facility following EPA’s revision to the toxicity estimate for ethylene oxide.
These 2018 activities included community ethylene oxide air monitoring and a risk assessment report completed by the Colorado Department of Public Health and Environment (CDPHE). As part of this effort, CDPHE conducted air monitoring at locations at the facility’s fence line and adjacent residential areas before and after Terumo BCT installed emission control measures to reduce fugitive emissions. CDPHE also evaluated ethylene oxide concentrations at several Denver-area monitoring locations to compare near-facility ethylene oxide concentrations with background concentrations not influenced by facility emissions. CDPHE’s analysis determined that control measures taken by Terumo BCT reduced community risk by 50-75%.
- Find more information on these activities, including monitoring and risk assessment reports.
- Learn more about the final rule for EtO sterilization facilities.
- For more information about actions you can take.
Watch the Community Meeting
A community meeting took place on September 22, 2022.
Please use this link or below to view: