IRIS Public Science Meeting (June 2016)
EPA hosted a public science meeting on June 29-30, 2016, the following draft assessment materials were discussed:
- IRIS Toxicological Review of tert-Butyl Alcohol (Public Comment Draft)
- Benzo[a]pyrene (BaP): Potential approaches to estimate the risk of skin cancer following dermal exposure.
Meeting Details
IRIS hosts public science meetings to allow the public the opportunity to provide input and participate in discussions about the draft IRIS assessment for tert-butanol and related materials to the BaP assessment [see Meeting Materials tabs].
Dates
The meeting was held on June 29-30, 2016.
Location
The meeting was held at the EPA Conference Center at 2777 South Crystal Drive, Arlington, Virginia 22202. The meeting was also available by webinar/teleconference for remote participants.
Meeting Agenda
Final meeting agenda (3 pp, 633 K) [Last Update Jun 22, 2016]
tert-butyl Alcohol (tert-Butanol) Meeting Materials
- IRIS Toxicological Review of tert-Butanol (Public Comment Draft) (140 pp, 3 MB)
- Supplemental Information on the IRIS Toxicological Review of tert-Butanol (Public Comment Draft) (107 pp, 2 MB)
- Public comment draft of the peer review charge for reviewers (5 pp, 174 K)
- Tables of evidence pertaining to kidney effects in animals exposed to ETBE (18 pp, 1 MB)
- All references sorted by author (tert-butanol)(dynamic literature link - generated by HERO)
- Literature selection process for tert-butanol(generated by HERO)
- Provide comments on these materials in the tert-butanol docket at EPA-HQ-ORD-2013-0111.
- Federal Register Notice: May 16, 2016 announcing the availability of the public comment draft of tert-butanol and the 60-day public comment period.
Key Science Topics
-
Science Topic 1: Mode of action for thyroid follicular cell tumors
Lifetime oral exposure to tert-butyl alcohol (tert-butanol) has been associated with increased thyroid follicular cell adenomas in female B6C3F1 mice and increased thyroid follicular cell adenomas and carcinomas in male B6C3F1 mice (NTP, 1995). An antithyroid MOA has been evaluated for the thyroid tumors observed following tert-butanol exposure in mice. This MOA involves increased clearance of thyroid hormones by the liver, which may cause continual secretion of TSH by the pituitary, leading to follicular cell hyperplasia and tumors (U.S. EPA, 1998a). EPA conducted an MOA analysis and found that the available database was inadequate to draw any conclusions regarding an antithyroid MOA.
The public comment draft of the Toxicological Review for tert-butyl Alcohol (tert-Butanol) discusses the synthesis of thyroid effects in Section 1.2.2. Further evaluation of carcinogenicity is provided in Section 1.3.2.
The IRIS Program is seeking public discussion on this or other possible modes of action and their relevance to the observed thyroid effects. -
Science Topic 2: Disentangling mechanisms of kidney toxicity and carcinogenicity.
tert-Butanol is a metabolite of ethyl tertiary butyl ether (ETBE) and has been shown to mediate several kidney effects observed following ETBE exposure (Salazar et al., 2015), thus both chemical databases (i.e., tert-butanol and ETBE) provide information relevant to understanding the mechanisms underlying kidney toxicity and carcinogenicity. Reported effects following tert-butanol and ETBE exposure include changes in kidney weight, histopathological endpoints, and serum markers of kidney toxicity. α2u-Globulin was detected in the hyaline droplets of male rats following tert-butanol and ETBE exposure. While tert-butanol meets the criteria to indicate that an α2u-globulin process is operating and some of the kidney effects are not relevant to humans, the data were insufficient to conclude that α2u-globulin nephropathy is the sole contributor to tert-butanol-induced renal tumors; ETBE did not induce renal tumors. The observed renal effects in male and female rats following exposure to tert-butanol and ETBE are also associated with chronic progressive nephropathy (CPN). Several of the CPN pathological effects are similar to, and can obscure the lesions characteristic of, α2u-globulin-related hyaline droplet nephropathy (Webb et al., 1990). Additionally, CPN effects can be exacerbated by both chemical exposure as well as renal effects of α2u-globulin accumulation (U.S. EPA, 1991). The underlying mechanisms regulating CPN and its exacerbation are not well understood. For example, there is no scientific consensus on the role of CPN in rat kidney carcinogenesis (Melnick et al., 2012; Hard et al., 2013; Hard et al., 2009).
The public comment draft of the IRIS Toxicological Review for tert-butyl Alcohol (tert-Butanol) presents a summary of kidney toxicity and carcinogenicity evidence in Section 1.2.1 with additional discussion in Section 1.3.2. Because ETBE induces similar effects, all kidney evidence tables and figures from the Toxicological Review for ETBE are also being released to supplement the discussion.
The IRIS Program is seeking discussion on the roles that α2u-globulin nephropathy and CPN play in the observed kidney toxicity and carcinogenicity of tert-butanol.
References
- Hard, GC; Johnson, KJ; Cohen, SM. (2009). A comparison of rat chronic progressive nephropathy with human renal disease-implications for human risk assessment [Review]. Crit Rev Toxicol 39: 332-346.
- Hard, GC; Banton, MI; Bretzlaff, RS; et al. (2013). Consideration of rat chronic progressive nephropathy in regulatory evaluations for carcinogenicity. Toxicol Sci 132: 268-275.
- Melnick, R; Burns, K; Ward, J; Huff, J. (2012). Chemically Exacerbated Chronic Progressive Nephropathy Not Associated with Renal Tubule Tumor Induction in Rats: An Evaluation Based on 60 Carcinogenicity Studies by the National Toxicology Program. Toxicol Sci 128: 346-356.
- National Toxicology Program (NTP). (1995). Toxicology and carcinogenesis studies of t-butyl alcohol (CAS no 75-65-0) in F344/N rats and B6C3F1 mice (Drinking water studies). NTPTR436. National Toxicology Program. Research Triangle Park: NC. 305 pp.
- Salazar, KD; Brinkerhoff, CJ; Lee, JS; Chiu, WA. (2015). Development and application of a rat PBPK model to elucidate kidney and liver effects induced by ETBE and tert-butanol. Toxicol Appl Pharmacol 288: 439-452.
- U.S. EPA (U.S. Environmental Protection Agency). (1991). Alpha-2u-globulin: Association with chemically induced renal toxicity and neoplasia in the male rat. (EPA/625/3-91/019F). Washington, DC: U.S. Environmental Protection Agency, Office of Research and Development.
- U.S. EPA (U.S. Environmental Protection Agency). (1998a). Assessment of thyroid follicular cell tumors [EPA Report]. (EPA/630/R-97/002). Washington, DC.
- Webb, DR; Kanerva, RL; Hysell, DK; Alden, CL; Lehman-McKeeman, LD. (1990). Assessment of the subchronic oral toxicity of d-limonene in dogs. Food and Chem Toxicol 28: 669-675.
Notes
In 2013, the IRIS Program began to host public science meetings to seek input from the public and to foster scientific discussion of draft materials for IRIS assessments under development.
For this public comment draft, the IRIS Program has identified two science topics where further public discussion will aid in revising the draft assessment and submitting it for peer review.
The public is invited to provide written comments on these and other science topics specific to this draft assessment. In addition, there will be an opportunity to provide oral comments during a separate session at the meeting.
Providing Comments
Provide comments on the key science topics in the tert-butanol docket at EPA-HQ-ORD-2013-0111.
Previously Released Materials
Materials previously released for this assessment may be found on the tert-butanol chemical landing page.
Benzo[a]pyrene (BaP)
Background
Benzo[a]pyrene, or BaP, causes skin cancer in mice following dermal exposure; in fact, dermal exposure studies of other chemicals often use benzo[a]pyrene as a positive control for carcinogenicity. Humans are dermally exposed to benzo[a]pyrene through contaminated soil and sediment, and the EPA’s program and regional offices have expressed a need for toxicity values for dermally active chemicals, such as benzo[a]pyrene. To meet this EPA need, in September 2014 the EPA released a draft IRIS Toxicological Review of Benzo[a]pyrene that included an approach for estimating the risk of skin cancer following dermal exposure. This was the first time a health agency has developed and proposed a solution to this problem.
In April 2016, the EPA’s Science Advisory Board Chemical Assessment Advisory Committee (SAB CAAC) released their peer-review advice on the 2014 draft IRIS Benzo[a]pyrene assessment. Their report made several recommendations, including that the extrapolation from mice to humans be supported by a more coherent, logical structure. They also recommended that skin cancer risk be modeled as a function of absorbed dose rather than applied dose of benzo[a]pyrene.
The IRIS Program is initiating a public science discussion on this topic in order to obtain a broad range of scientific perspectives on how to approach this problem.
Data for estimating lifetime risks from skin cancer in mice following dermal exposure
The EPA’s 2014 draft IRIS Toxicological Review of Benzo[a]pyrene reviewed the known carcinogenicity studies of benzo[a]pyrene administered to mice via dermal exposure. After excluding less than lifetime studies, studies that did not test multiple doses of benzo[a]pyrene, and studies only with dose levels inducing 90−100% incidence of tumors, 10 studies remained (see Table 1-16 of the 2014 draft IRIS Benzo[a]pyrene assessment for information on the design and results of these studies).
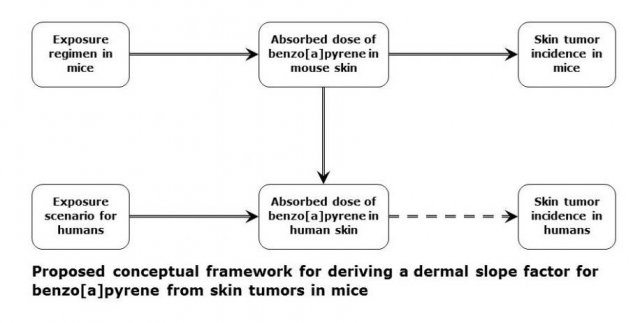
Proposed conceptual framework
The challenge of estimating the potential risk of human skin cancer from dermal exposure to benzo[a]pyrene can be broken down into several steps:
- Determine the appropriate units for expressing absorbed dose.
- Develop a model of absorbed dose as a function of the experimental exposure regimen in mice.
- Scale absorbed dose between mouse skin and human skin.
- Model the incidence of skin tumors in mice as a function of absorbed dose, scaled to humans.
Key Science Topics
- Determining appropriate dose-metrics for expressing absorbed BaP dose.
The CAAC noted that both total mass of benzo[a]pyrene and mass per unit of skin area have been used as dose metrics in previous publications (Knafla et al., 2011; 2006; Hussain et al., 1998; LaGoy and Quirk; 1994; Sullivan et al., 1991), and that “there does not appear to be any empirical data available to inform a choice between these two dose metrics or to select another.” Whichever dose metric is selected, it will need to be paired with an appropriate exposure equation for estimating an average daily dose of benzo[a]pyrene absorbed into human skin (an example equation can be found on p. G-14 of the supplemental information for the 2014 draft IRIS Benzo[a]pyrene assessment).
The IRIS Program is seeking public input on points that are important to consider and would be informative in selecting a dose metric.
- Modeling absorbed dose as a function of exposure parameters.
The CAAC noted that for the mouse study used to derive the dermal slope factor, applied dose closely approximates absorbed dose. The CAAC described conditions where this would be the case: the mass of the chemical is too small to cover completely the application area, the time between dose applications is long, and metabolism in the viable epidermis (the target tissue) is not saturated. In experimental studies where applied dose does not closely approximate absorbed dose, it would be important to estimate the absorbed dose from the exposure parameters in the study.
The CAAC also recommended that for subsequent use of the dermal slope factor to estimate the human cancer risk from an environmental exposure, the cancer risk should be estimated from the absorbed dose, and that the absorbed dose should be estimated from the exposure scenario.
Thus, there are recommendations to estimate absorbed dose both for an experimental exposure regimen in mice and for an environmental exposure scenario for humans.
The IRIS Program is seeking public input on factors to consider in developing a model of absorbed dose of benzo[a]pyrene as a function of the parameters of applied dose.
- Scaling absorbed dose between mouse skin and human skin.
After consideration of limitations in the available toxicokinetic data, EPA selected allometric scaling (i.e., body-weight3/4) and presented alternative approaches in the supplemental information for the 2014 draft IRIS benzo[a]pyrene assessment (see Appendix E). The CAAC noted that the science is uncertain for choosing the best approach for scaling absorbed dose from mouse skin to human skin. It is unknown whether whole-body toxicokinetics using allometric scaling is the most appropriate model within the skin compartment. The CAAC recommended consideration of thickness of the viable epidermis and metabolic rates in this tissue.
The IRIS Program is seeking public input on factors, with particular attention to quantitative factors, to better inform a scaling approach from mouse skin to human skin.
References
- Hussain, M; Rae, J; Gilman, A; Kauss, P. (1998). Lifetime health risk assessment from exposure of recreational users to polycyclic aromatic hydrocarbons. Arch Environ Contam Toxicol 28 35: 527-531.
- Knafla, A; Petrovic, S; Richardson, M; Campbell, J; Rowat, C. (2011). Development and 3 application of a skin cancer slope factor for exposures to benzo[a]pyrene in soil. Regul 4 Toxicol Pharmacol 59: 101-110.
- Knafla, A; Phillipps, KA; Brecher, RW; Petrovic, S; Richardson, M. (2006). Development of a dermal cancer slope factor for benzo[a]pyrene [Review]. Regul Toxicol Pharmacol 45: 7 159-168.
- LaGoy, PK; Quirk, TC. (1994). Establishing generic remediation goals for the polycyclic aromatic hydrocarbons: Critical issues [Review]. Environ Health Perspect 102: 348-352.
- Sullivan, MJ; Miller, CJ; Custance, SR. (1991). A risk assessment for crude oil in residential surface soils. In EJ Calabrese; PT Kostecki (Eds.), Hydrocarbon contaminated soils, v I: remediation techniques, environmental fate, risk assessment, analytical methodologies, regulatory considerations. Chelsea, MI: Lewis Publishers, Inc.
Notes
In 2013, the IRIS Program began to host public science meetings to seek input from the public and to foster scientific discussion of draft materials for IRIS assessments under development.
The IRIS Program is proposing three science topics where further public discussion will aid in revising its approach to estimating the risk for skin cancer following dermal exposure to benzo[a]pyrene. In the future, the revised approach will be submitted for public comment and peer review.
Providing Comments
Provide comments on the key science topics in the BaP docket at EPA-HQ-ORD-2011-0391.
Previously Released Materials
Related materials may be found on the benzo[a]pyrene chemical landing page.
In addition, the final SAB peer review report may be found on the SAB website (SAB CAAC Review – Draft Toxicological Review of Benzo[a]pyrene).
