Understanding the Science of Ocean and Coastal Acidification
On this page:
Ocean Acidification
The Industrial Revolution’s Effect on the Global Carbon Cycle
Until recently, the amount of carbon dioxide in the atmosphere has fluctuated slightly and slowly during the past 10,000 years. However, the Industrial Revolution of the 1700s started a global adoption of fossil fuels to power human activity. The rate at which fossil fuels like coal, oil, and natural gas are burned has increased up until the present day. Burning fossil fuels releases carbon dioxide gas to the atmosphere, and the ever-increasing global use of fossil fuels has caused the amount of carbon dioxide in the atmosphere to increase to a concentration that is higher than any time in the past 800,000 years. The cutting of forests for fuel or to clear land for agriculture over the past 250 years has also contributed to higher carbon dioxide levels in the atmosphere because trees capture and store carbon dioxide via photosynthesis.
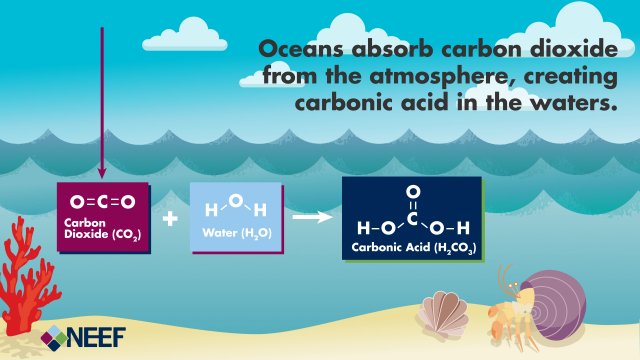
Not only do higher atmospheric concentrations of carbon dioxide alter the Earth’s climate, they also impact ocean chemistry. This is because carbon dioxide in the atmosphere readily dissolves into water.
Dissolved Carbon Dioxide: Gases in Liquid?
Carbon Dioxide Imparts Acidity: Transformations of Carbon Dioxide in Water
Once carbon dioxide dissolves in water, it reacts with water molecules to form carbonic acid . Carbonic acid can be further transformed to bicarbonate and carbonate ions. These four different forms of carbon (dissolved carbon dioxide, carbonic acid, bicarbonate, and carbonate) exist in balanced proportions in seawater. As more carbon dioxide is added to seawater, the balance shifts and the carbonate ion concentration decreases as it is transformed to bicarbonate due to increasing acidity.
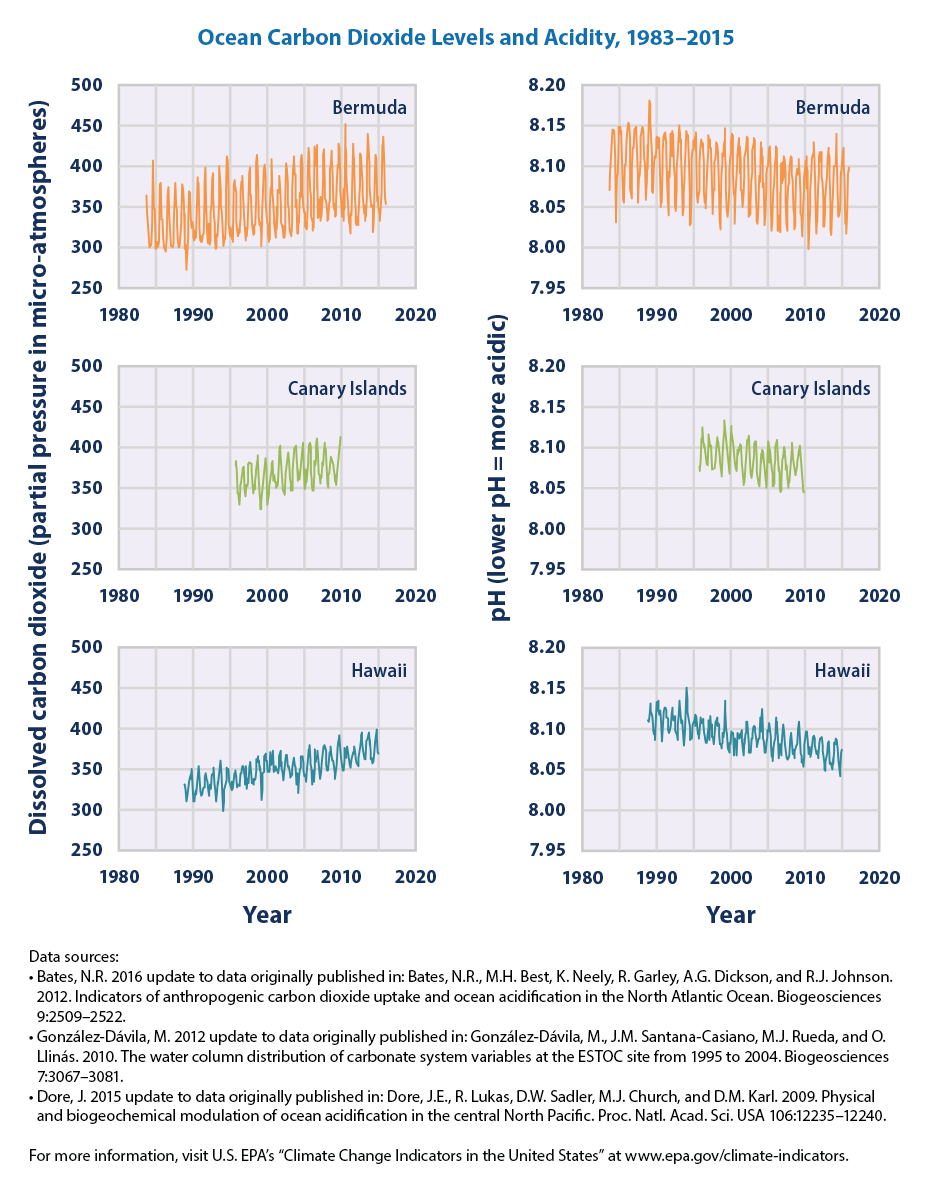
How Acidity is Measured: pH
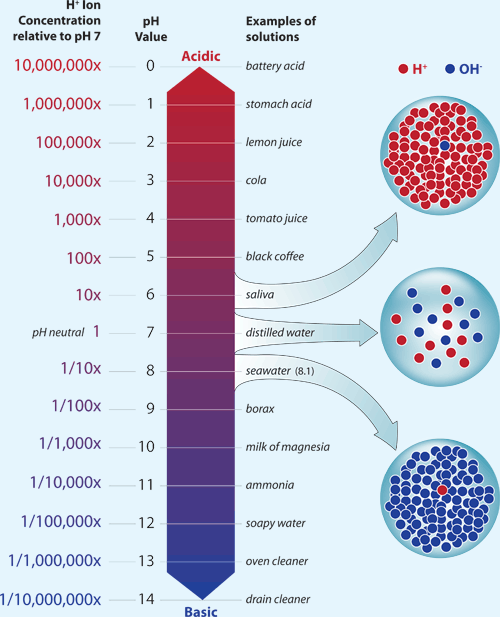
The acidity of a liquid is reported as pHA representation of hydrogen ion concentration (molar hydrogen ion concentration to the negative base 10 logarithm) . The lower the pH value, the higher the acidity of a liquid. Solutions with low pH are acidic and solutions with high pH are basic (also known as alkaline).Prior to the Industrial Revolution, average ocean pH was about 8.2. Today, average ocean pH is about 8.1. This might not seem like much of a difference, but the relationship between pH and acidity is not direct. Each decrease of one pH unit is a ten-fold increase in acidity. This means that the acidity of the ocean today, on average, is about 25% greater than it was during preindustrial times.
Acidity and Availability of Shell-forming Calcium
Carbonate
Marine life uses carbonate from the water to build shells and skeletons. As seawater becomes more acidic, carbonate is less available for animals to build shells and skeletons. Under conditions of severe acidification, shells and skeletons can dissolve.
Coastal Acidification
Closer to Home: Coastal Acidification
Human activity also contributes to acidification in coastal waters. Acid-forming compounds (including carbon dioxide) are released
Acid Rain
Burning fossil fuels for energy releases water and carbon dioxide as the main byproducts, but nitrogen oxides and sulfur dioxide are also released in smaller amounts. These two acid-forming compounds fall back to Earth’s surface. They can land in coastal waters directly, or more often mix with water in the atmosphere before falling as acid rain. Acid rain typically has a pH between 4.2 and 4.4.
Excess Nutrients Delivered Via Streams
The elements nitrogen and phosphorus are essential nutrients for living things. For this reason ,farmers homeowners, and gardeners supply nitrogen and phosphorus to crops, lawns, and gardens to stimulate plant growth. However, water can carry excess nutrients down streams and into coastal waters. Agricultural activities are a major source of nutrients to coastal waters, but other sources include