RadTown Radon Activity 4: The Half-Life of Radon
Radon is a radionuclide, which means that, as time passes, it undergoes radioactive decay. Every radionuclide has a half-life, which is the time it takes for half of the radioactive particles present to decay away. In this activity, students will learn about radioactive decay and decay chains. This activity is intended for middle and high school students.
- Objectives
- Next Generation Science Standards
- Materials and Resources
- Time
- Vocabulary
- Directions
- Common Core State Standards
- Printable Worksheets and Classroom Aids
Objectives
Students will:
- Learn about radioactive decay, decay chains, and how radon forms radioactive products or particles that can be harmful.
- Demonstrate the concept of half-life.
- Calculate and chart the half-life of a given sample.
- Discuss the significance of knowing the half-life of radioactive elements.
Next Generation Science Standards
The concepts in this activity can be used to support the following science standards:
- PS1. Structure and Properties of Matter
- ESS3. Earth and Human Activity
Materials and Resources
Each italicized document title can be found at the bottom of this page, and is available for printing and distribution.
- Radon: Teacher Background Information
- Vocabulary Materials
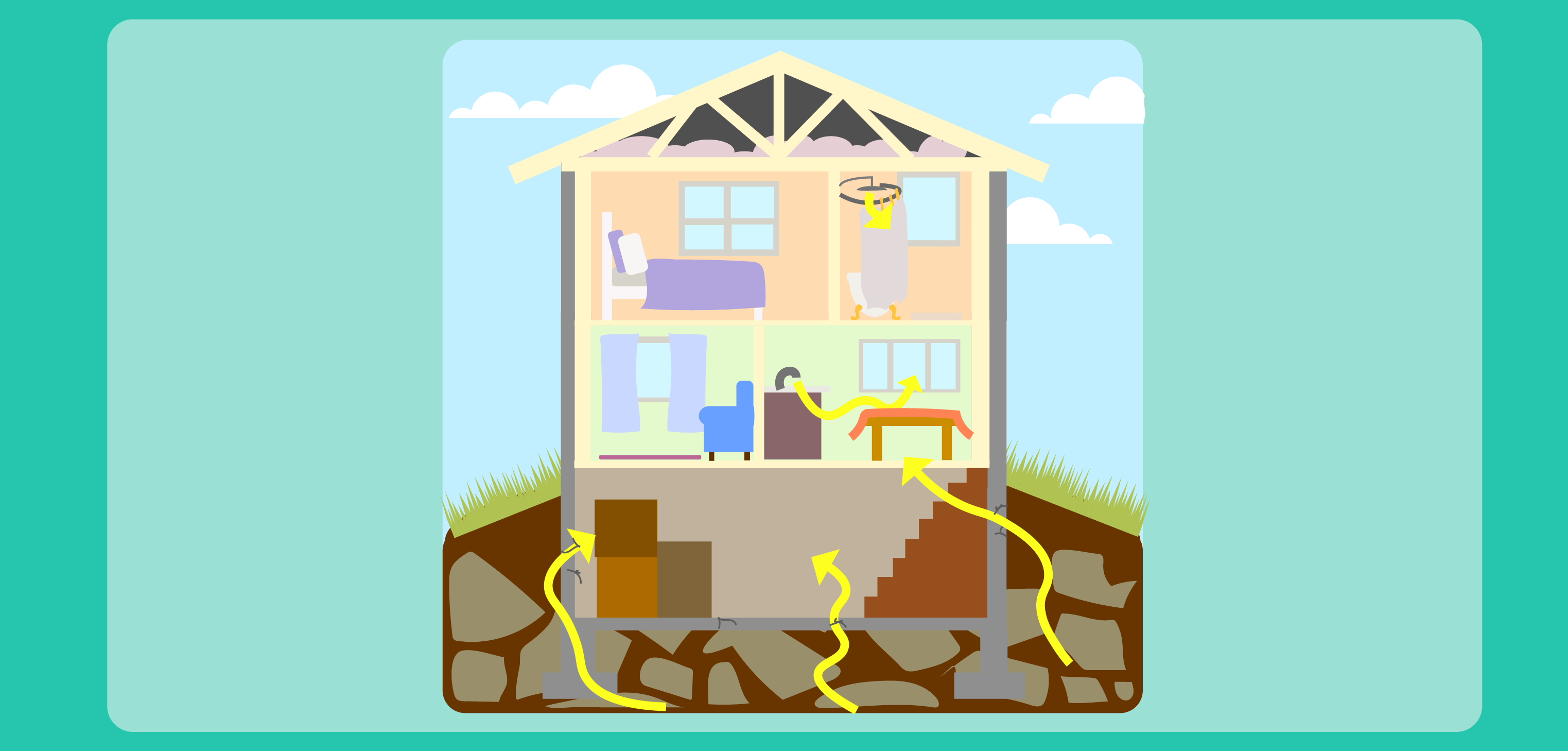
- Radon Exposure image (display or copy for students)
- Computer and projector for displaying information
- Half-Life Data Sheet (one per student, pair or group) and Half-Life: Teacher Answer Key
- Student calculators (optional)
- Radon-222 Decay Chain (display or copy for students)
Time
45-60 minutes, not including optional activities or extensions.
Vocabulary
- Ionizing radiation
- Radiation
- Radon
- Uranium
Directions
- Start with a vocabulary activity if students are not familiar with radon and the terms used in this activity.
- Display the Radon-222 Decay Chain image. Explain that radioactive elements are made up of atoms with an unstable nucleus. As the nucleus tries to become stable, it gives off (emits) energy or radiation in the form of alpha particles, beta particles, gamma radiation or a combination of the three. When radiation is emitted, the nucleus forms a new nucleus of a different element. This process continues until the nucleus becomes stable and is no longer radioactive.
Radon is a radioactive gas. Its decay products (polonium, bismuth and lead) are metals that can easily attach to dust and other particles in the air. Those particles can be circulated and transported in air and inhaled. When radon is found in buildings and in the environment, there are thousands and thousands of atoms that contribute to the overall concentration of radon in a space. Even though, individually, an atom will give off energy in a single burst, because there are thousands of atoms, it appears that the radon atoms are emitting radiation continuously. These bursts of energy, or emissions of radiation, are what scientists and technicians measure in the environment. .
The energy that radon and its decay products give off (emit) as they decay is what can cause people to develop cancer. This energy can damage cells and cause them to become cancerous. This type of radiation is called “ionizing radiation”.
Ionizing radiation has so much energy it can knock electrons out of atoms, a process known as ionization. Ionizing radiation can affect the atoms in living things, so it poses a health risk by damaging tissue and DNA in genes.
- Explain that each radioactive element has a half-life. Half-life is the amount of time it takes for half of the radioactive atoms in a sample to decay into a more stable form. In the decay chain, students can see that radon has a half-life of 3.8 days. Polonium-218 has a half-life of 3 minutes.
- Provide students with the Half-Life Data Sheet. Have them read the initial statement and form a hypothesis.
- Demonstrate the concept of half-life with the class by choosing from the following options:
- Option A: Select three volunteers. Have the volunteers stand at a distance from an easily identifiable location (e.g., a wall or the classroom door). Direct each volunteer to move at varying rates (fast, moderate and slow) to represent half-lives of different elements. For example, radon has a half-life of 3.8 days, radium has a half-life of 1600 years, and uranium has a half-life of 4.5 billion years. Direct each volunteer to walk halfway toward the identifiable location at their designated rate and stop before continuing to the next halfway point between them and the identifiable location. They will continue this process until they cannot go any farther. You can mark the halfway points with string or paper if students need the guidance.
- Option B: Ask for 12 volunteers. Have the volunteers line up in the front of the room. Provide each volunteer with two different colored sheets of paper to represent radon and polonium. Have all of the volunteers hold the radon paper out front facing the students. Have half of the volunteer (any 6 of the 12) place the polonium colored paper out front to represent half of the atoms that transformed to polonium. Then have the next half (3 of the 6 volunteers showing radon) place the polonium paper out front. In the next half-life, have one volunteer place the polonium paper out front and have another volunteer show half radon and half polonium by folding one or both of the papers in half. The remaining volunteer should then place the polonium paper out front and the volunteer showing half radon, half polonium should fold one or both papers to represent ¾ radon and ¼ polonium.
- Option C: Show an online video or demonstration of half-life. Sources may include TeacherTube, other allowed Internet sources, or Colorado University’s online applet that demonstrates half-life and radioactive decay.
- Direct students to complete the remainder of the Half-Life Data Sheet. The use of calculators is optional.
- Ask students to share their observations and conclusions from the activity. The Half-Life Teacher Answer Key is provided.
- Conclude by sharing that radon is naturally present in our world. The chances of getting lung cancer from radon depends mostly on:
- The indoor radon level of the places where a person spends most of their time. The U.S. Environmental Protection Agency (EPA) recommends fixing or lowering radon levels that are 4 picocuries per liter (pCi/L) or higher.
- The amount of time a person is exposed to higher levels of radon.
- Whether a person is also exposed to cigarette smoke (which can also cause lung cancer).
- Optional activities or extensions:
- Have students create posters for the National Radon Poster Contest
- Draw a diagram of lungs or locate a video showing the progression of lung cancer as the lungs go from healthy to damaged over a span of years.
- Construct a model of the lungs providing a breakaway of the lung to show its inner tissue (bronchioles, alveoli and bronchial tubes) to show how radon products can stick to lung tissue and cause damage.
- Invite a nurse, doctor or health care professional to come in and talk about radon exposure and lung cancer.
The concepts in The Half-Life of Radon activity align with the following:
- CCSS English Language Arts Standards for Literacy in History/Social Studies, Science, & Technical Subjects
- CCSS.ELA-LITERACY.SL.6-12.1 Comprehension and Collaboration
- CCSS.ELA-LITERACY.SL.6-12.2 Comprehension and Collaboration
- CCSS.ELA-LITERACY.SL.6-12.5 Presentation of Knowledge and Ideas
- CCSS.ELA-LITERACY.RST.6-12.7 Integration of Knowledge and Ideas
- CCSS.ELA-LITERACY.WHST.6-12.1 Text Types and Purposes
- CCSS Mathematics Standards
- CCSS.MATH.PRACTICE.MP1